Solutions
Our Product Hub was designed to provide a comprehensive overview of the Joyhann portfolio of products . With product overviews,visual imagery, features and benefits, product spec information, and an array of support content, we are here to support you.
Delivering trusted performance, built on truly open architectures
We offer a full spectrum of tailored infection control solutions that optimize healthcare workflows, enhance patient safety, and improve operational efficiency across hospitals, research institutions, and laboratories globally.

Laboratory Hygiene & Safety
Anasys champions a culture of safety that transcends compliance, embedding protection into the very fabric of laboratory operations. Our philosophy prioritizes human well-being and environmental integrity, ensuring every researcher works with confidence and security. By integrating robust engineering controls and advanced protective systems, we create environments where scientific innovation flourishes without compromising safety. This commitment reflects our foundational belief that true progress is built on a bedrock of unwavering safety and care for both people and specimens.
Integrated Biosafety Engineering and Protection Systems
Anasys implements a layered defense strategy utilizing Class II/III Biosafety Cabinets, negative pressure systems, and HEPA filtration. We integrate advanced PPE, automated decontamination, and continuous environmental monitoring. Our solutions include emergency safety equipment, validated maintenance protocols, and real-time control systems, ensuring comprehensive containment and compliance with international biosafety standards across all laboratory environments.


Laboratory Information System
Anasys envisions the Laboratory Information System as the digital nervous system of modern healthcare, transforming raw data into diagnostic intelligence. We empower laboratories to achieve operational excellence and enhanced patient safety through seamless data integration and intelligent workflow automation. Our systems foster a collaborative ecosystem where information flows effortlessly, supporting clinicians in making timely, informed decisions and elevating the standard of care through reliable, accessible diagnostic insights
Architected Data Management and Workflow Automation
Anasys LIS features core modules for patient, order, and specimen management,with robust instrument interfacing and middleware integration. It supports HL7 standards, automated quality control with Westgard rules, and advanced analytics.The system ensures regulatory compliance, offers customizable reporting, and enables efficient resource tracking within a secure, role-based access architecture.


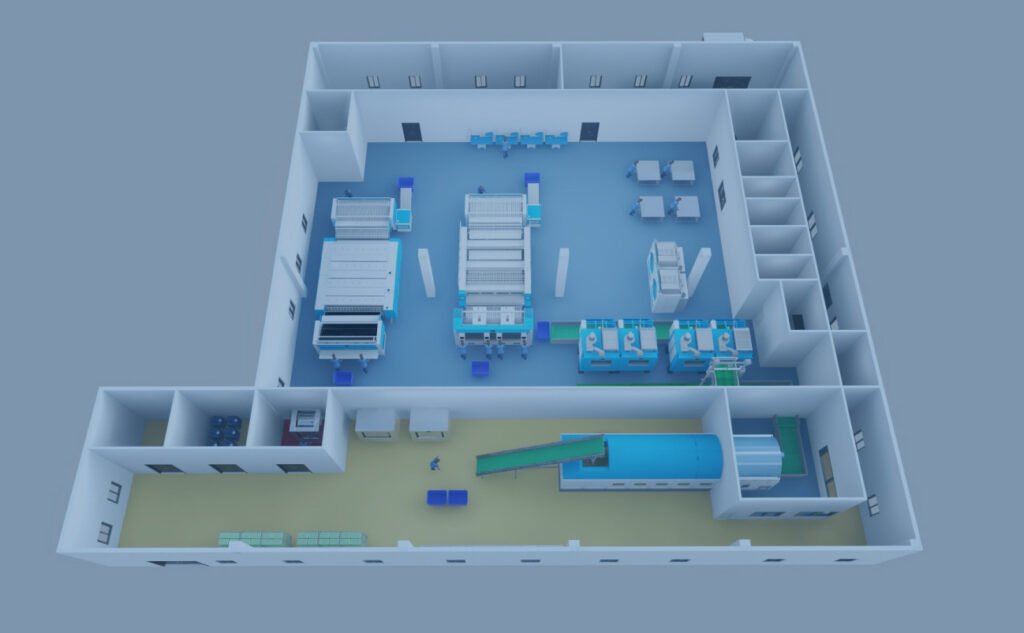
Large-Volume Laboratory
Anasys designs large-volume laboratories as symphonies of diagnostic excellence,where scale harmonizes with precision. We create centralized hubs that process thousands of samples daily, delivering comprehensive testing from routine to esoteric. Our approach ensures that efficiency, accuracy, and innovation converge,providing healthcare networks with reliable, high-speed diagnostic services that form the cornerstone of effective patient management and advanced medical research.
High-Throughput Automation and Specialized Workflow Zoning
Anasys engineers facilities with Total Laboratory Automation (TLA), integrating pre analytical sorters, automated tracks, and analytical modules. The design enforces unidirectional workflow, specialized sub-laboratory zoning (e.g., Molecular, Flow Cytometry), and critical infrastructure redundancy. This ensures maximum throughput, minimal turnaround time, and stringent quality control for a vast test menu.

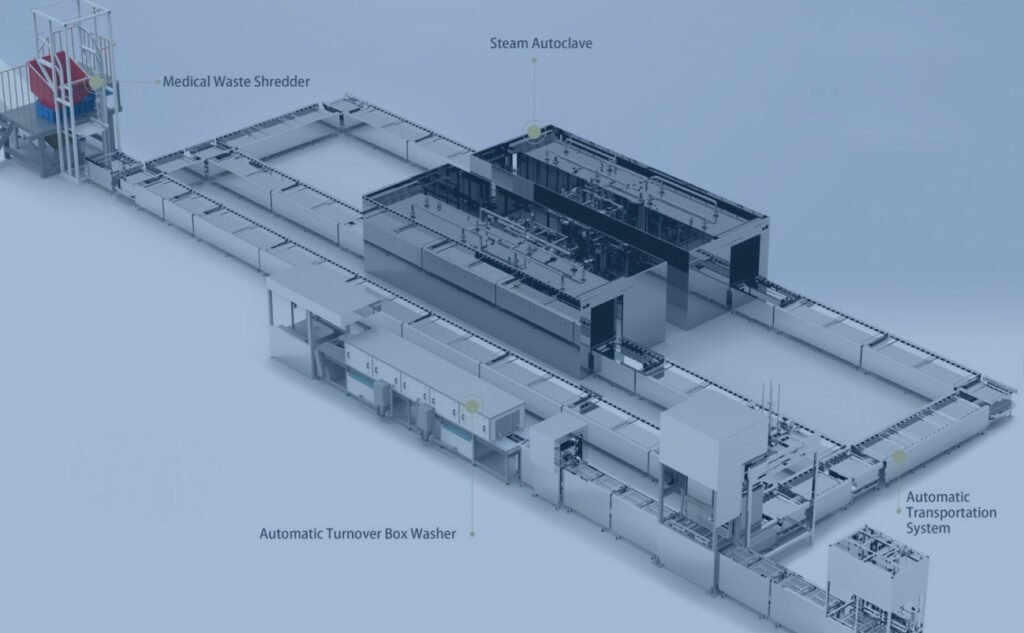
Local Reagent Production
Anasys pioneers a transformative shift towards diagnostic self-sufficiency with localized reagent production. We empower hospitals to reclaim control over their supply chain, ensuring uninterrupted diagnostic services even during global disruptions. Our vision is one of quality sovereignty and operational resilience, where institutions can tailor formulations to their unique needs, fostering a new era of reliability, cost-efficiency, and customized diagnostic solutions.
cGMP-Compliant Manufacturing and Aseptic Filling
Anasys establishes in-house production units with ISO-classified cleanrooms, pharmaceutical-grade water systems, and precision mixing/filtration skids. The process encompasses raw material dispensing, bulk formulation, sterilizing filtration, and aseptic filling. Rigorous QC testing for conductivity, osmolality, and sterility ensures every batch meets exacting pharmacopeial standards.


Mid-Sized Laboratory
Anasys recognizes the mid-sized laboratory as a vital community nexus, balancing comprehensive diagnostics with operational agility. We build facilities that serve as primary diagnostic hubs, offering an expanded test menu without the overhead of large reference labs. Our solutions are crafted to deliver timely, accurate results that directly impact patient care, fostering trust and clinical collaboration within the communities we serve.
Optimized Workflow and Modular Analyzer Integration
Anasys designs mid-sized labs with zoned layouts for efficient sample flow. We integrate modular chemistry/immunoassay analyzers, automated hematology systems, and dedicated spaces for microbiology and specialized testing. The configuration includes a robust LIS, strategic pre-analytical automation, and managed reagent inventory to ensure operational efficiency and quality.
Small Laboratory
Anasys believes that proximity to care is paramount. Our small laboratory solutions bring essential diagnostics closer to the patient, dramatically reducing turnaround times for critical decisions in EDs and ICUs. We create compact, highly efficient spaces that embody the principle that impactful medicine often happens at the point of need, ensuring speed does not come at the expense of quality or safety.
Compact Core Analyzers and Strategic POCT Deployment
Anasys equips small labs with integrated hematology, chemistry, and coagulation analyzers. We strategically deploy Point-of-Care Testing (POCT) for ultra-rapid troponin and infectious disease screens. The design includes a Class II BSC, essential lab equipment, and a rigorous QA system, all within an ergonomic, safety-compliant footprint.


